FDA won't comment on status of Emergency Use Authorizations for two antibody treatments
Descrição
The US Food and Drug Administration told CNN Thursday morning that the agency doesn’t have any comments on the applications for Emergency Use Authorizations for Eli Lilly and Regeneron antibody treatments.
The coronavirus pandemic has brought countries to a standstill. In many places, as countries reopen, Covid-19 cases are on the rise. Follow here for the latest.
The coronavirus pandemic has brought countries to a standstill. In many places, as countries reopen, Covid-19 cases are on the rise. Follow here for the latest.

FDA pulls authorization for COVID antibody treatment over lack of
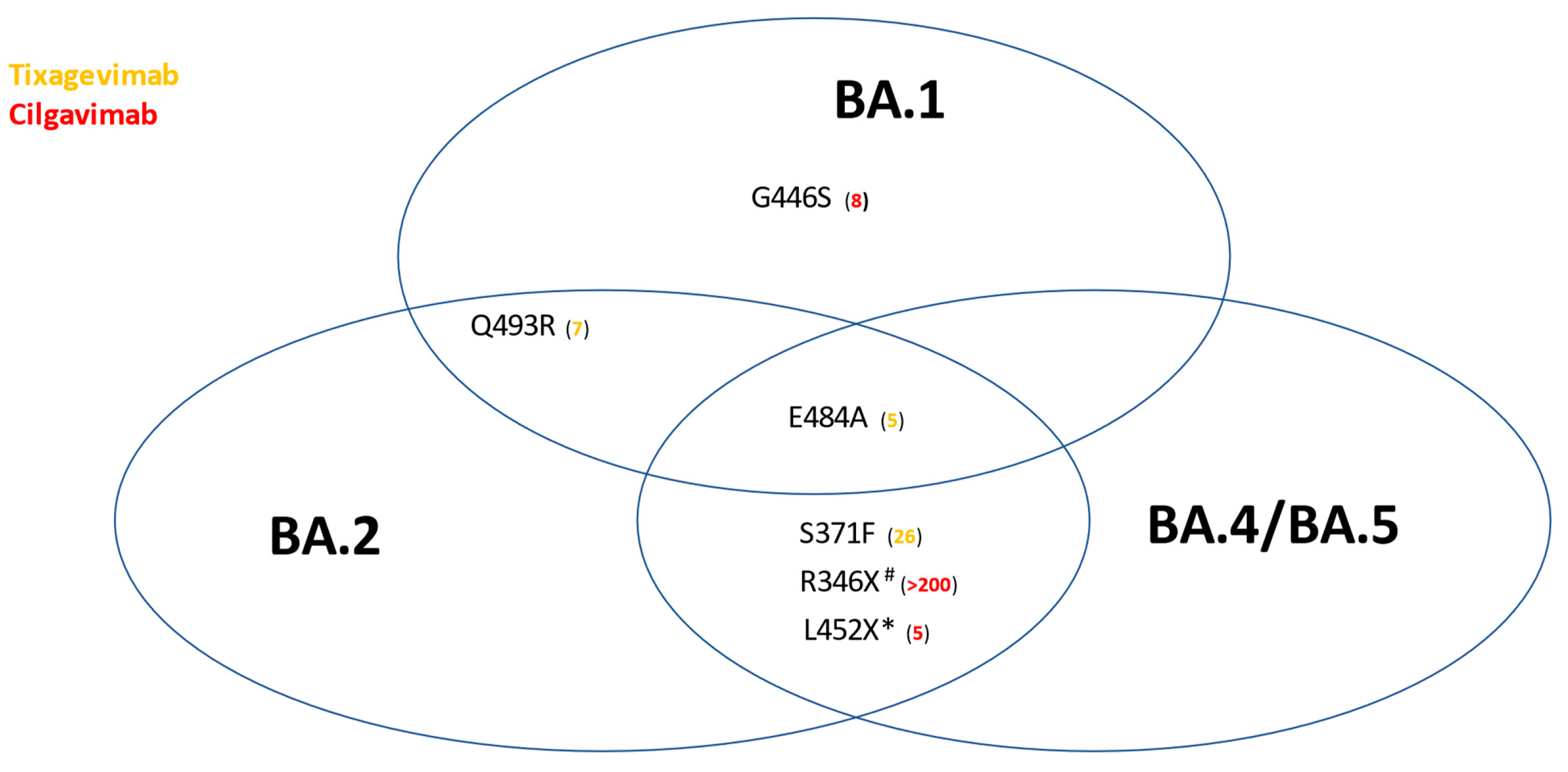
Viruses, Free Full-Text
FDA Meeting on COVID Vaccine for Children
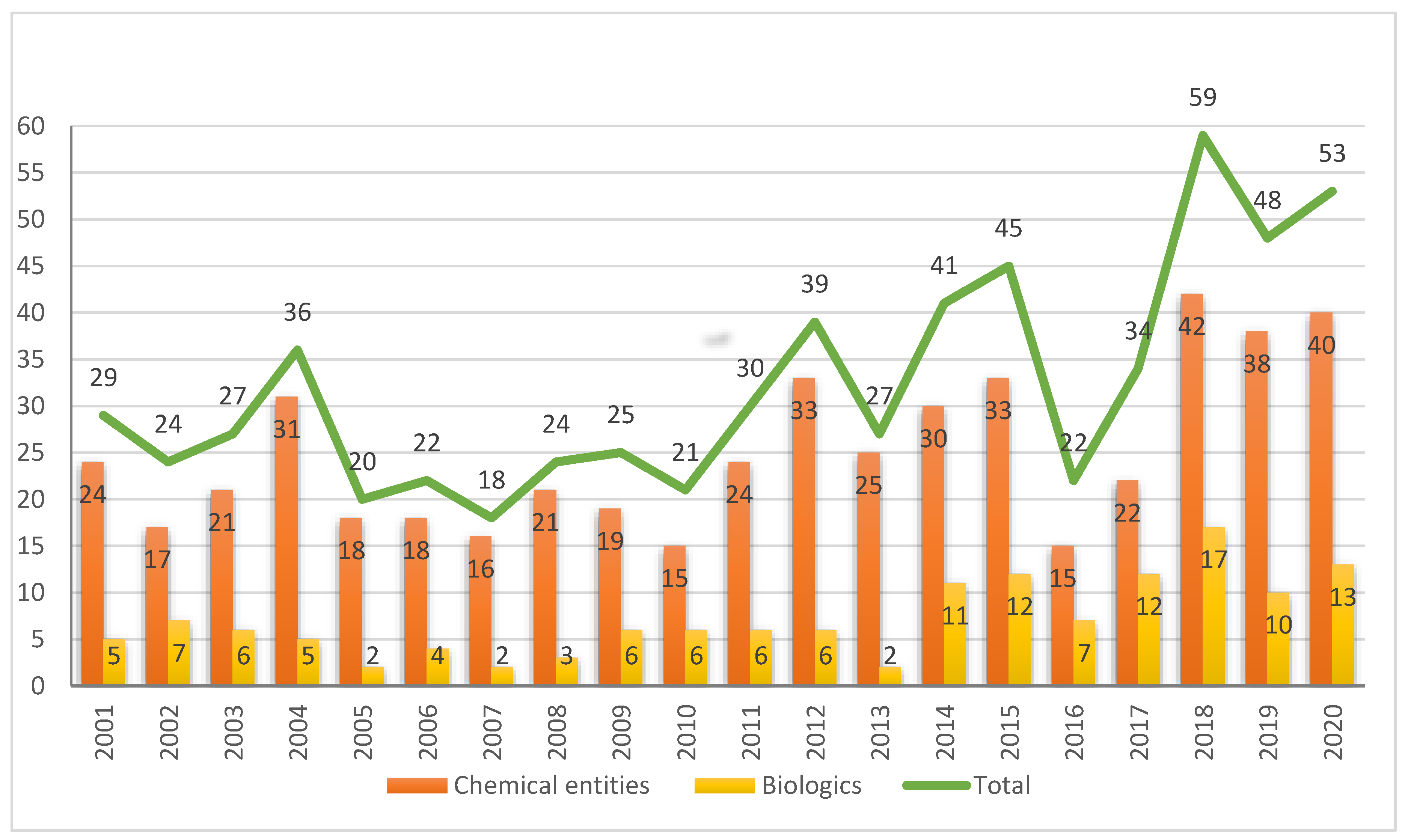
Molecules, Free Full-Text

Regulatory tracker: EMA backs Vertex's gene-editing therapy
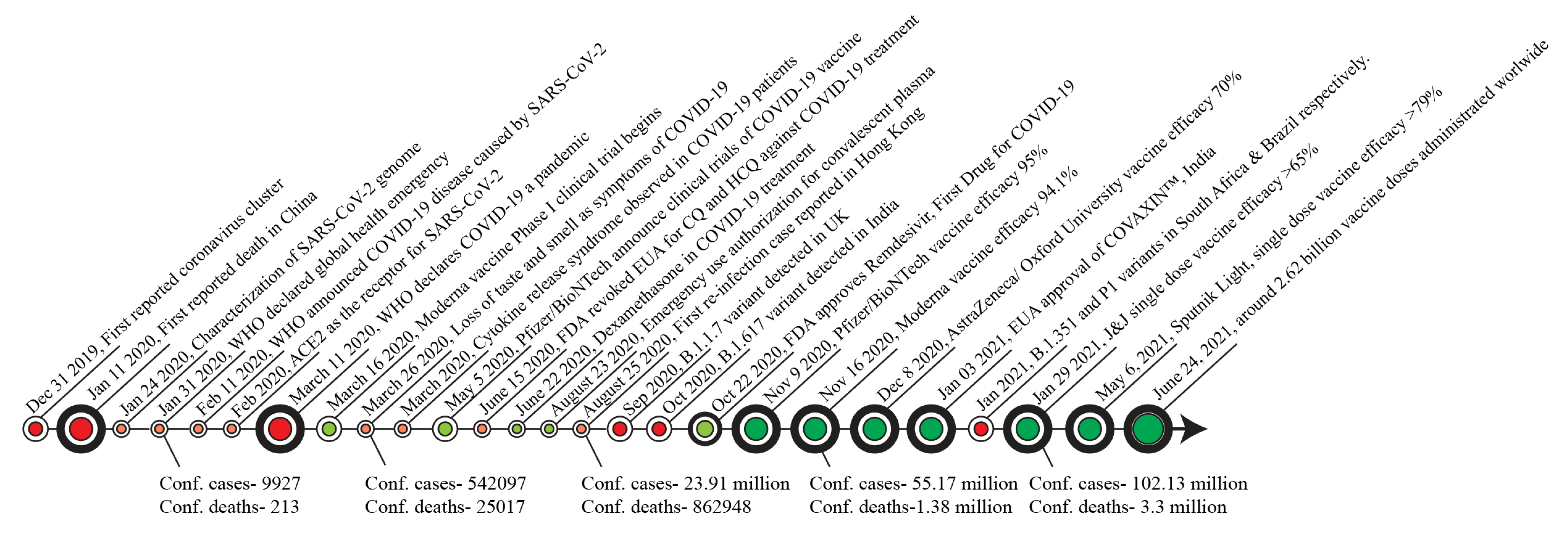
Biomolecules, Free Full-Text
Regeneron Covid-19 Drug REGEN-COV Prevents Infections for 8 Months
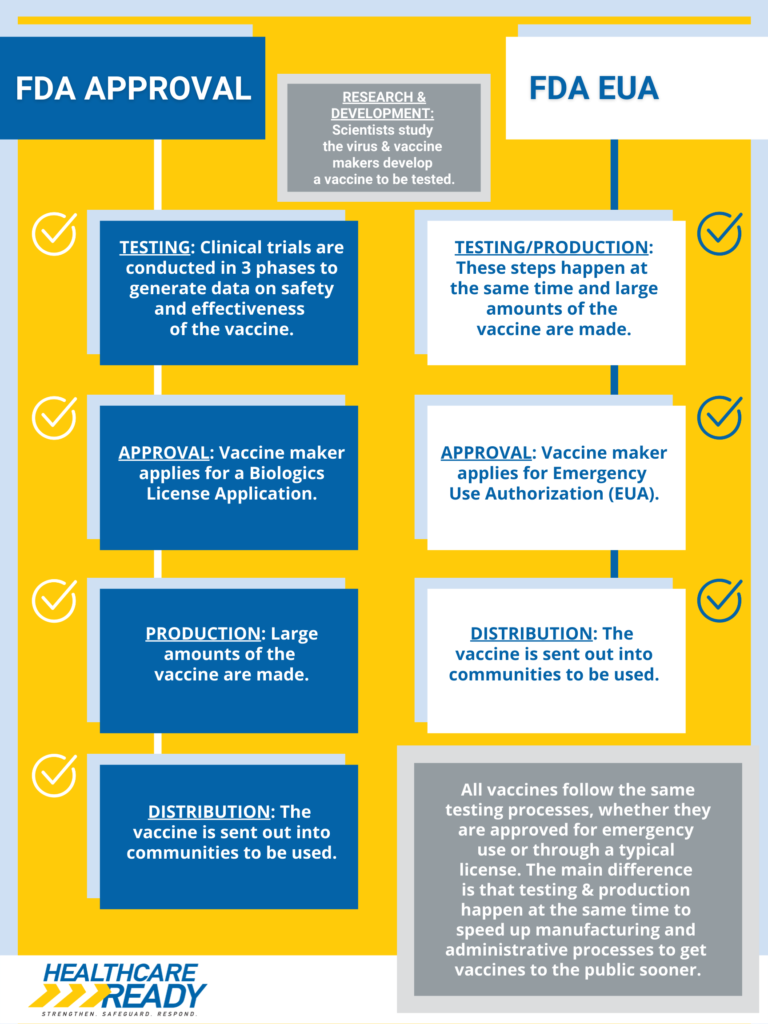
Infographic: FDA Approval vs. FDA Emergency Use Authorization

Moderna asks the F.D.A. to authorize its vaccine for children
de
por adulto (o preço varia de acordo com o tamanho do grupo)







