Top 4 eConsent Questions in Clinical Research: Forms & More
Descrição

eConsent
Top 4 eConsent Questions in Clinical Research: Forms & More
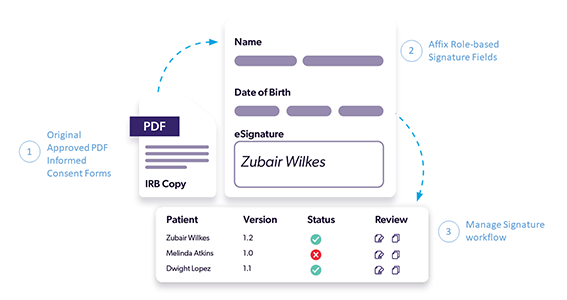
Scaling up eConsent
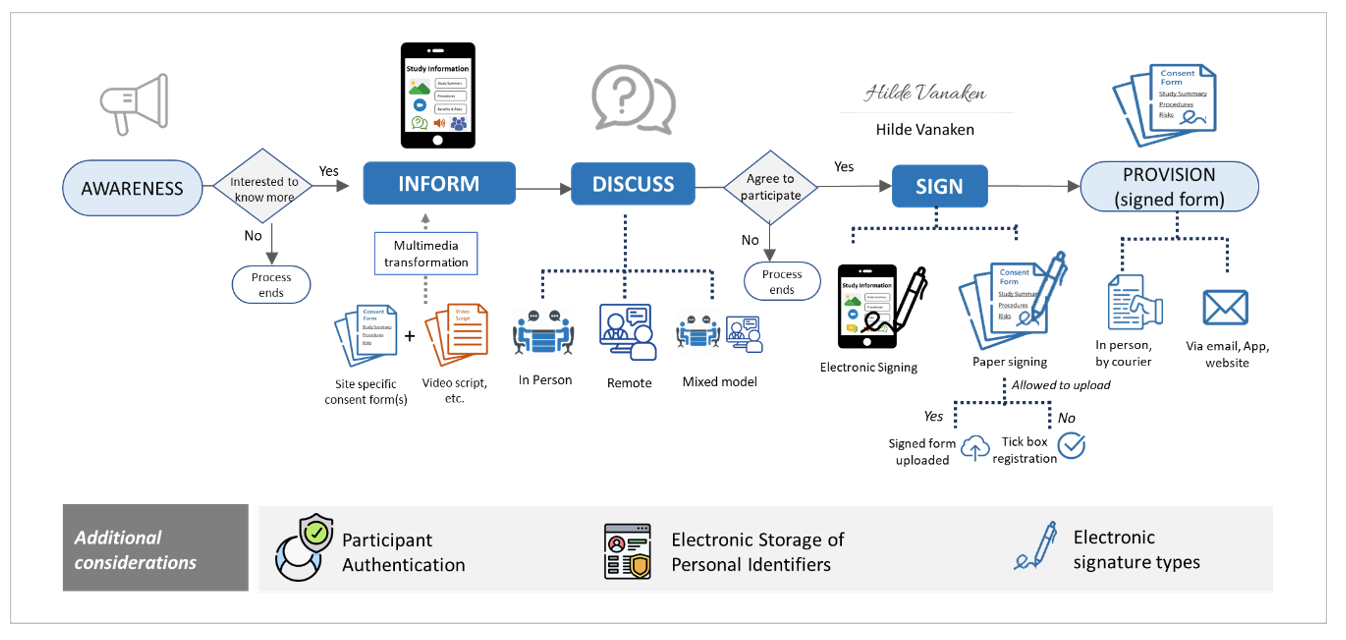
eConsent—The First Step to Enable Clinical Trial Access to Anyone
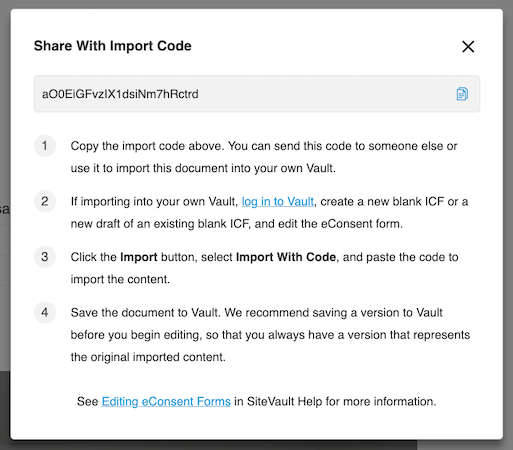
Using the Veeva eConsent Editor

eConsent—The First Step to Enable Clinical Trial Access to Anyone

E-Consent for Clinical Trials - Best Practices & Common Mistakes

Veeva eConsent, End-to-End eConsent Platform
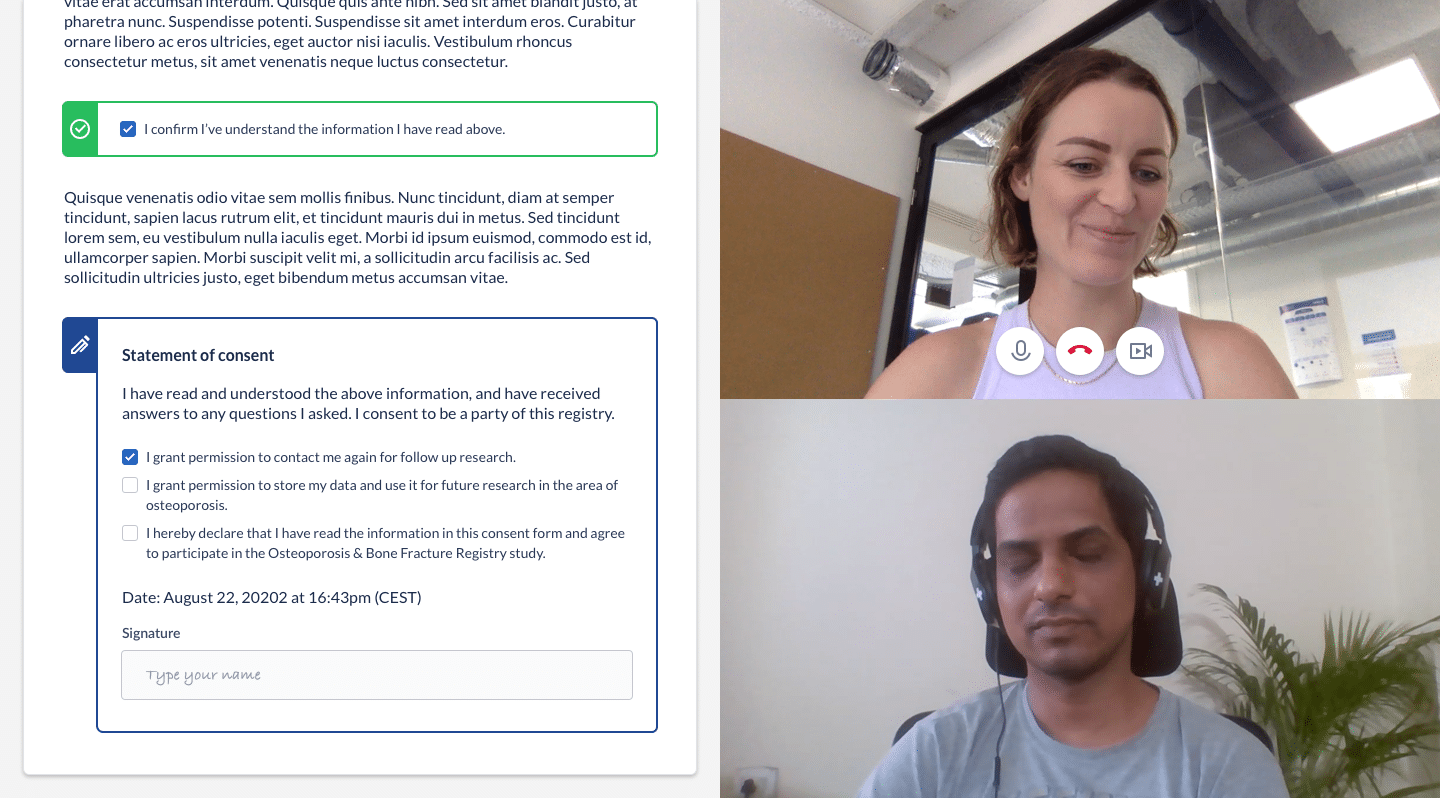
Top 4 eConsent Questions in Clinical Research: Forms & More

Top Considerations When Choosing eConsent Software - Florence

3 steps to more connected clinical trials - Today's Medical
eConsent in Clinical Trials
de
por adulto (o preço varia de acordo com o tamanho do grupo)