FDA OKs Cell Therapy to Lower Infection Risk After Stem Cell Transplant
Descrição
Omidubicel reduced infections in blood cancer patients from 60% to 39% at 100 days posttransplant

FDA Approves 2 New Bispecifics for Relapsed or Refractory Multiple

Delaying HSCT More Than 4 Weeks After COVID-19 Diagnosis May

Stem Cell Transplantation, Autologous Stem Cell Transplantation

One Expert's Approach in Transplant-Ineligible, Newly Diagnosed

How a stem cell treatment left patients feeling worse than before

Stem cell therapy for MS

Mesenchymal Stromal Cells: an Antimicrobial and Host-Directed

FDA Approves Cell Therapy for Blood Cancer Patients to Cut
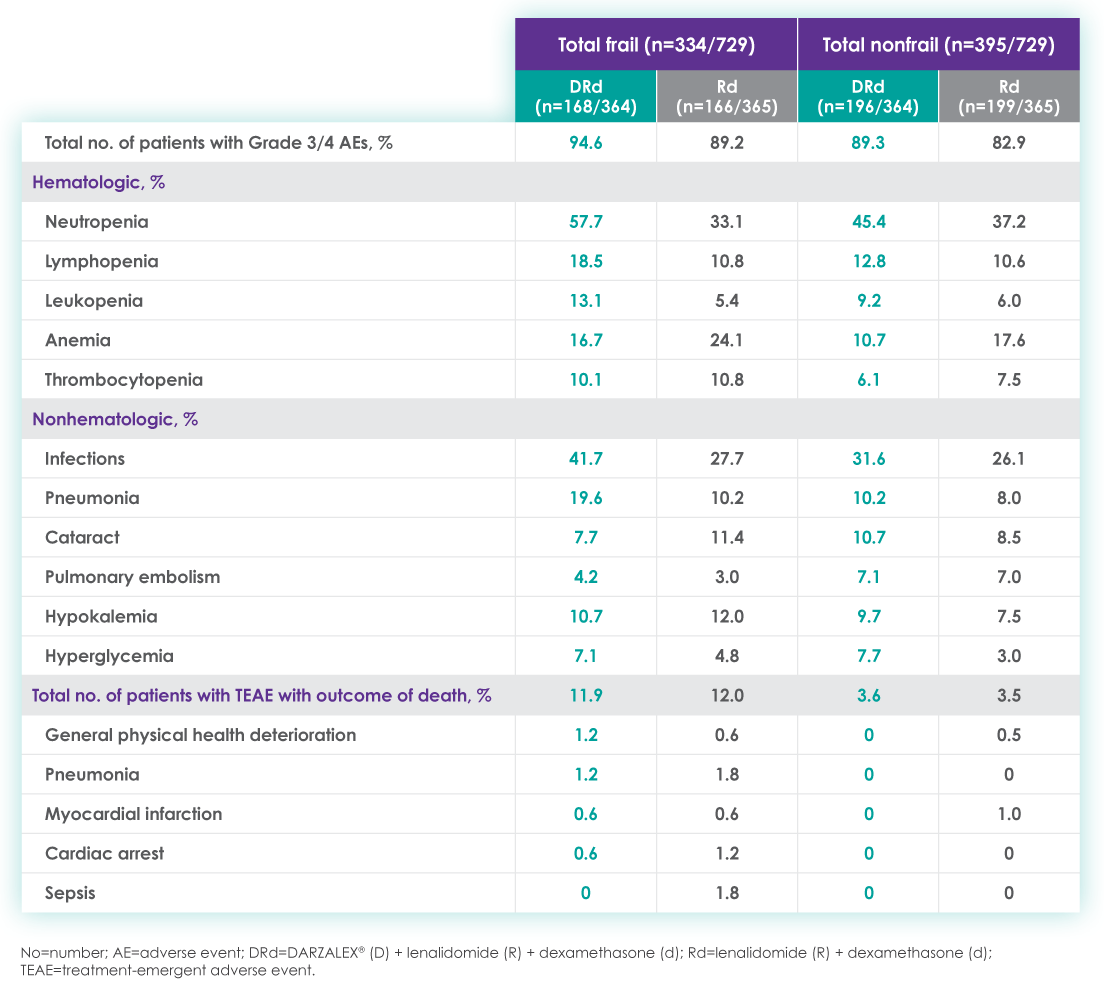
One Expert's Approach in Transplant-Ineligible, Newly Diagnosed

Mesenchymal Stromal Cells: an Antimicrobial and Host-Directed

Oncology/Hematology Leukemiaarticles page [1]

Adult Acute Lymphoblastic Leukemia Treatment (PDQ®) - PDQ Cancer

2014 Payers' Guide to New FDA Approvals by Dalia Buffery - Issuu
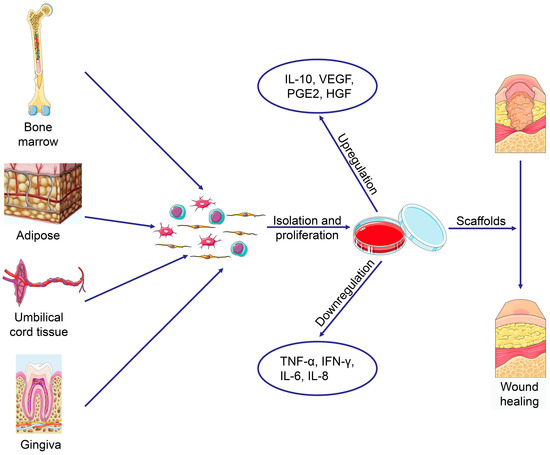
Cells, Free Full-Text

Critical review on the physical and mechanical factors involved in
de
por adulto (o preço varia de acordo com o tamanho do grupo)







