Nivolumab Plus Ipilimumab for Metastatic Castration-Resistant
Descrição
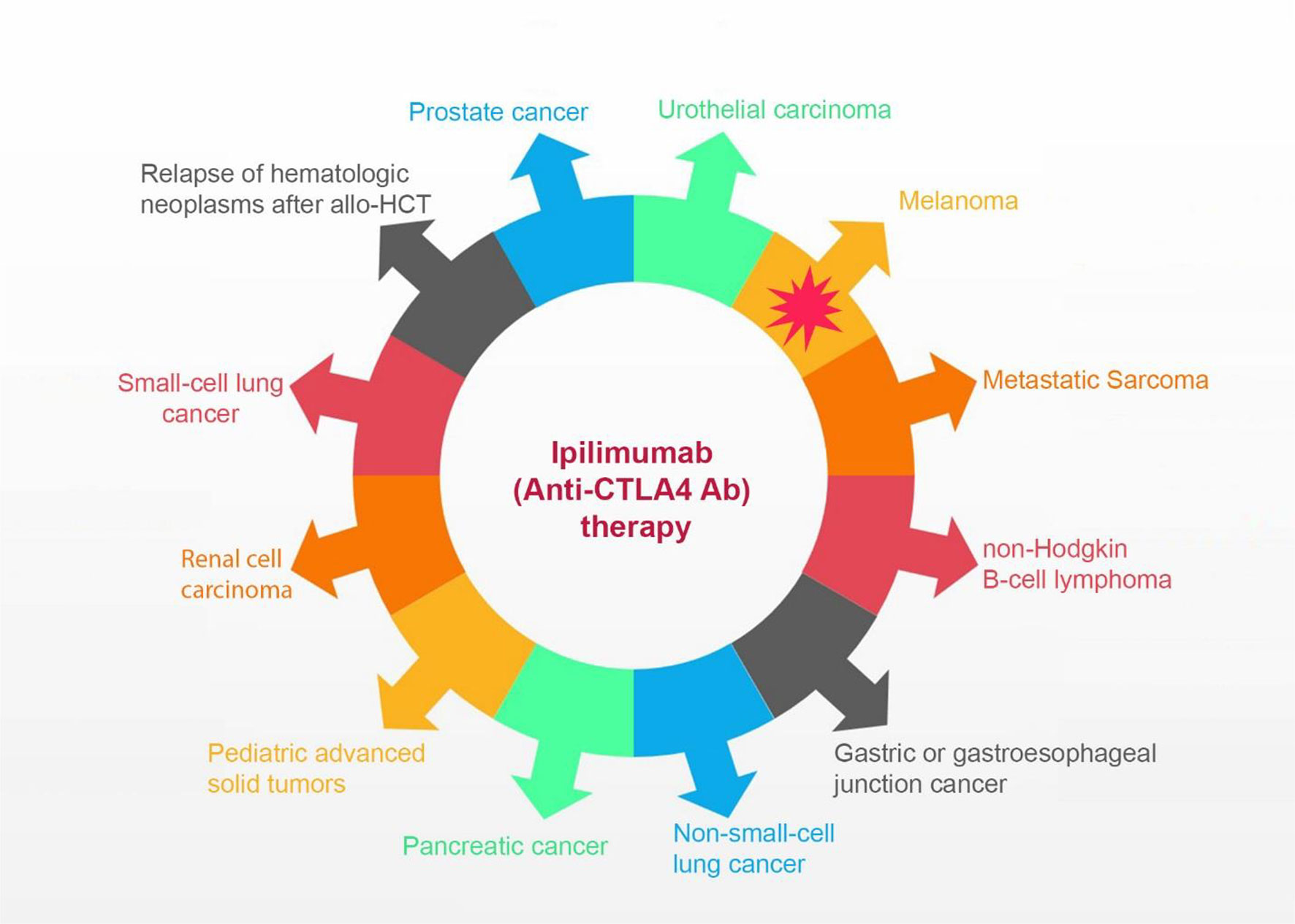
Frontiers Adverse Events Following Administration of Anti-CTLA4 Antibody Ipilimumab
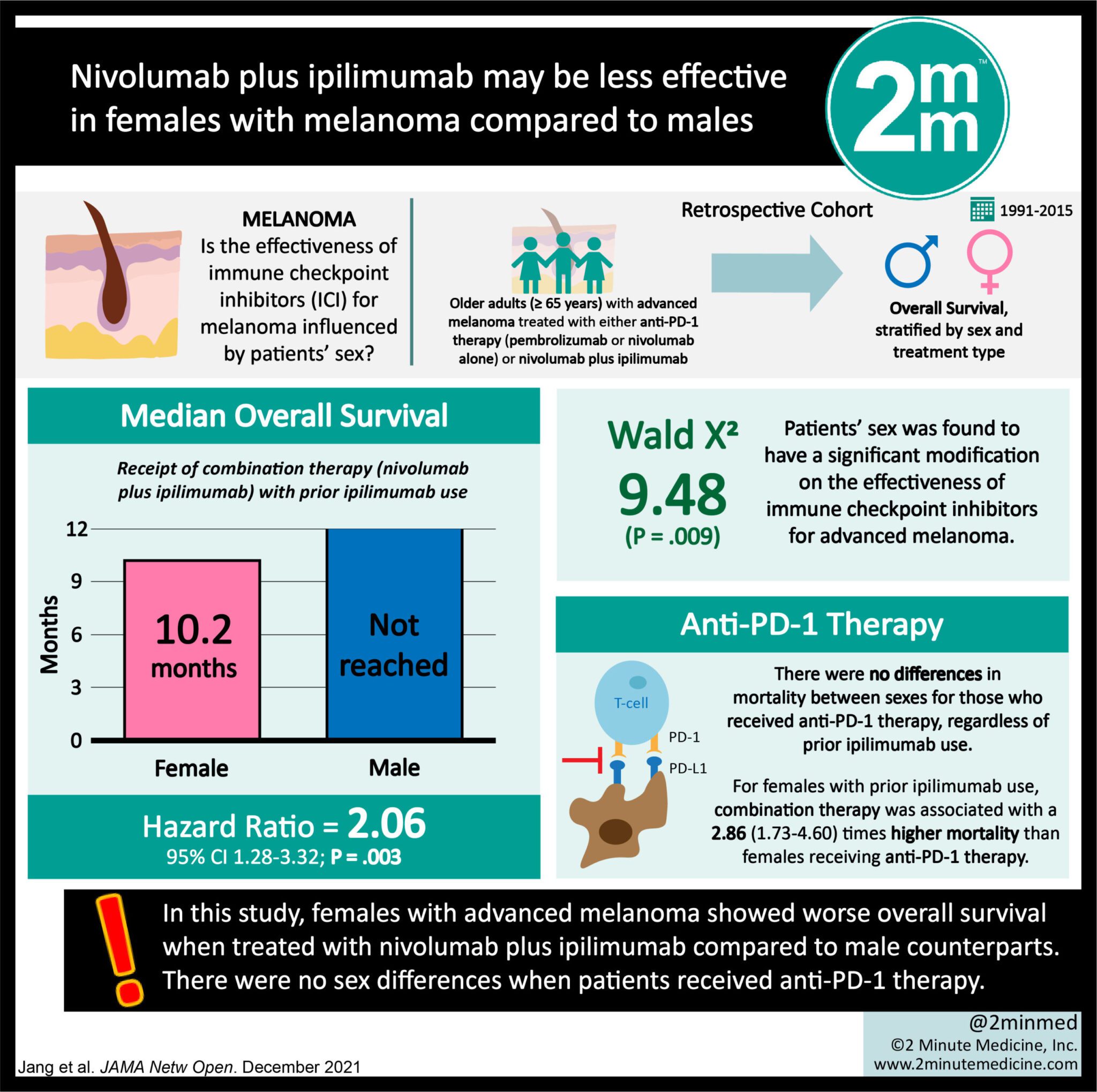
VisualAbstract: Nivolumab plus ipilimumab may be less effective in females with melanoma compared to males

A phase III, randomized, open-label study (CONTACT-02) of cabozantinib plus atezolizumab versus second novel hormone therapy in patients with metastatic castration-resistant prostate cancer
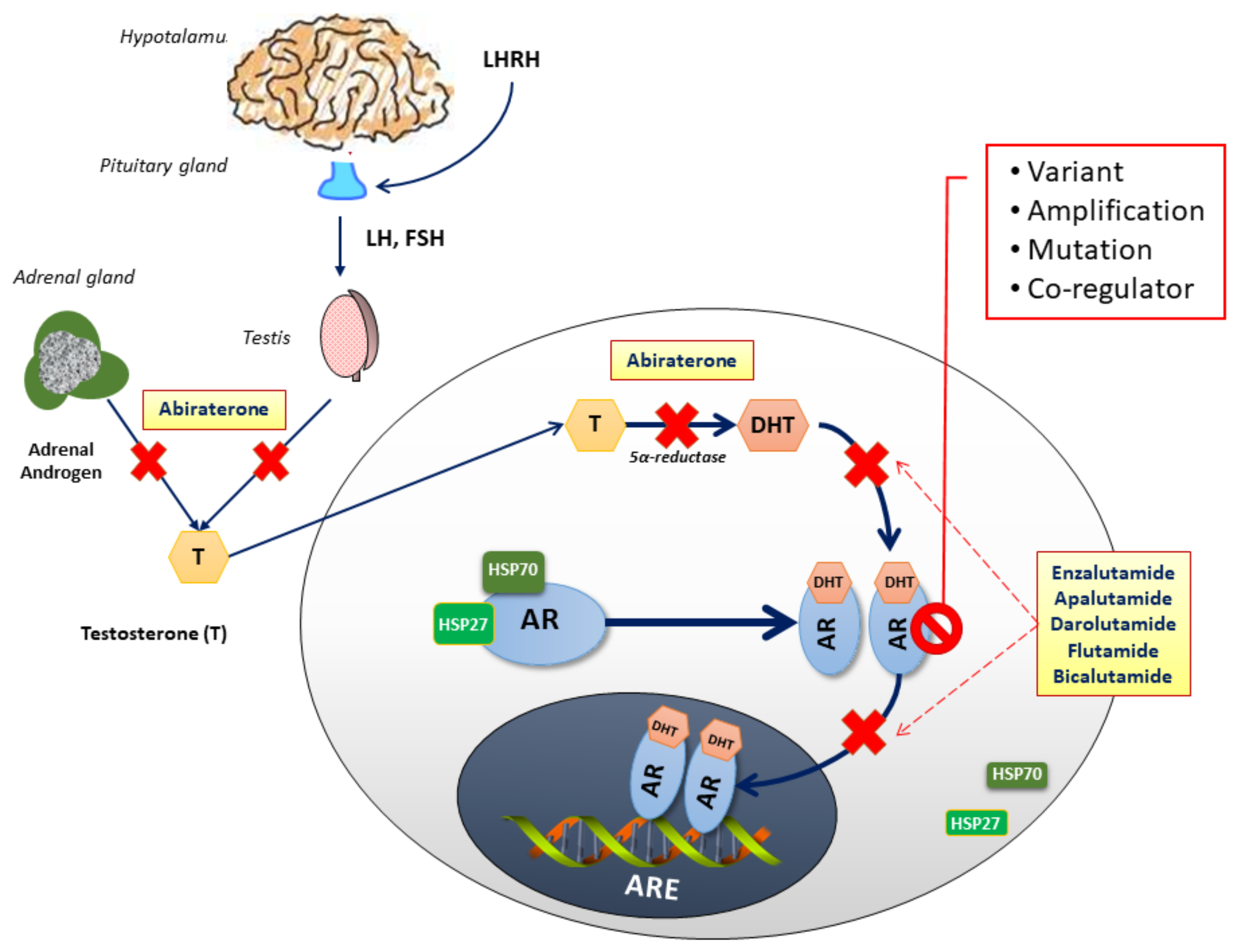
Cells, Free Full-Text

Nivolumab plus rucaparib for metastatic castration-resistant prostate cancer: results from the phase 2 CheckMate 9KD trial

New insights and options into the mechanisms and effects of combined targeted therapy and immunotherapy in prostate cancer: Molecular Therapy - Oncolytics

Safety and efficacy of avelumab plus carboplatin in patients with metastatic castration-resistant prostate cancer in an open-label Phase Ib study
PDF) Long-term complete remission with Ipilimumab in metastatic castrate- resistant prostate cancer: Case report of two patients
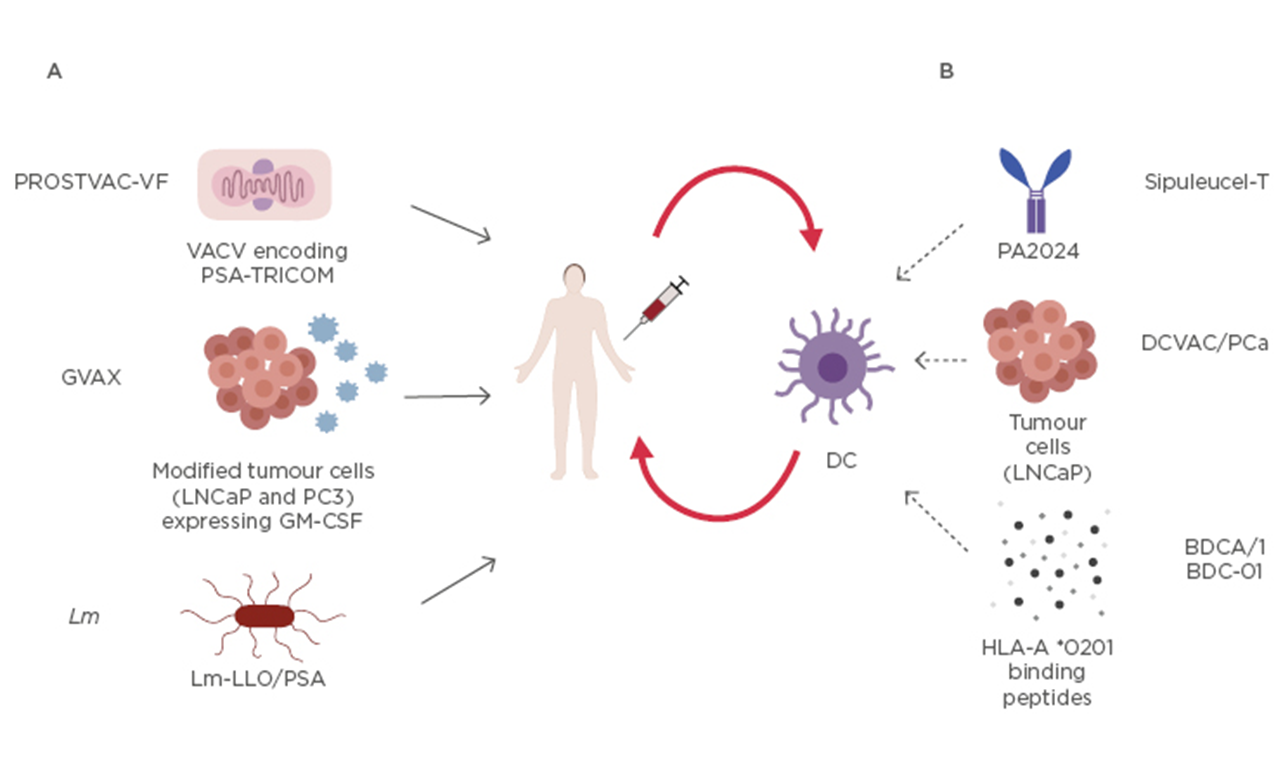
Immunotherapy in Prostate Cancer: Recent Advances and Future Directions - European Medical Journal
Nivolumab Plus Ipilimumab Demonstrates Anti-Tumor Activity in Metastatic Castration-Resistant Prostate Cancer

Single-dose 177Lu-PSMA-617 followed by maintenance pembrolizumab in patients with metastatic castration-resistant prostate cancer: an open-label, dose-expansion, phase 1 trial - The Lancet Oncology
de
por adulto (o preço varia de acordo com o tamanho do grupo)






