Hep B biotech Antios closed after FDA hold proved insurmountable
Descrição
Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable. | Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable.

Annalee Armstrong - Journalist Profile - Intelligent Relations

Former J&J R&D chief Mathai Mammen lands at FogPharma

Antios Therapeutics' ATI-2173 Demonstrates Suppression of

Core Concepts - Hepatitis B Coinfection - Co-Occurring Conditions

Microfluidic Formulation of Topological Hydrogels for Microtissue
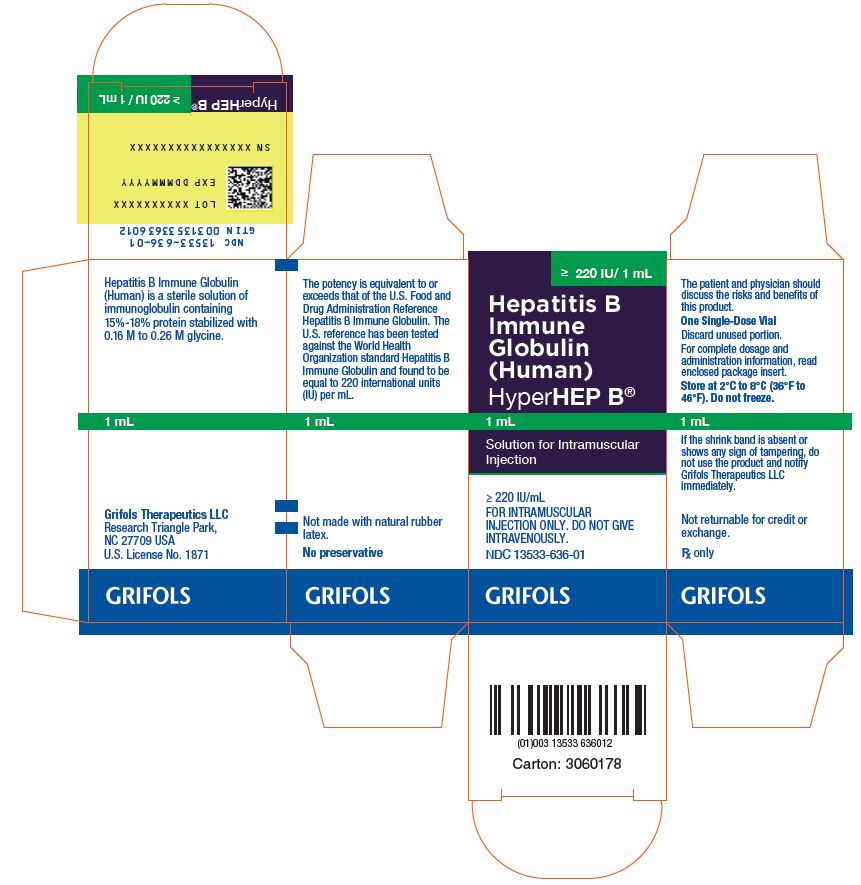
Hepatitis B Immune Globulin (Human) HyperHEP B®

Efficient long-term amplification of hepatitis B virus isolates

Hepatitis B Foundation

Biotech Fierce Biotech

Microfluidic Formulation of Topological Hydrogels for Microtissue

Hepatitis B Foundation
de
por adulto (o preço varia de acordo com o tamanho do grupo)