Ethide Laboratories - USP 88 In-Vivo Cytotoxicity Testing
Descrição
Learn what USP 88 cytotoxicity tests are available and which ones you will need to meet the regulatory requirements for your medical devices.

99 30943 360478 110230 598827223 PDF, PDF, Sterilization (Microbiology)

Championing In Vitro Biocompatibility/Biological Reactivity Testing for Plastics Used in Pharma Manufacturing
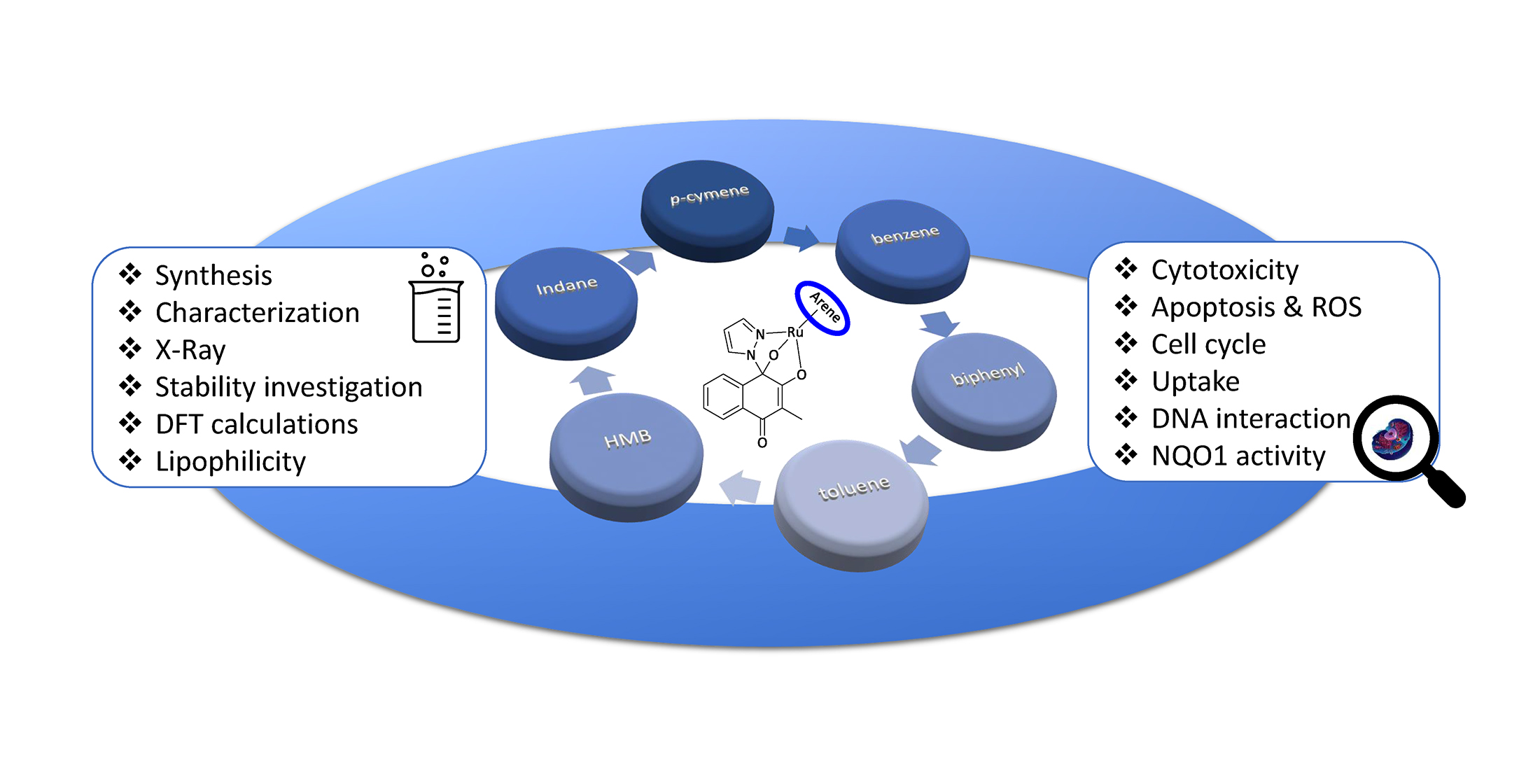
Pharmaceutics, Free Full-Text

Ethide Laboratories - USP 88 In-Vivo Cytotoxicity Testing
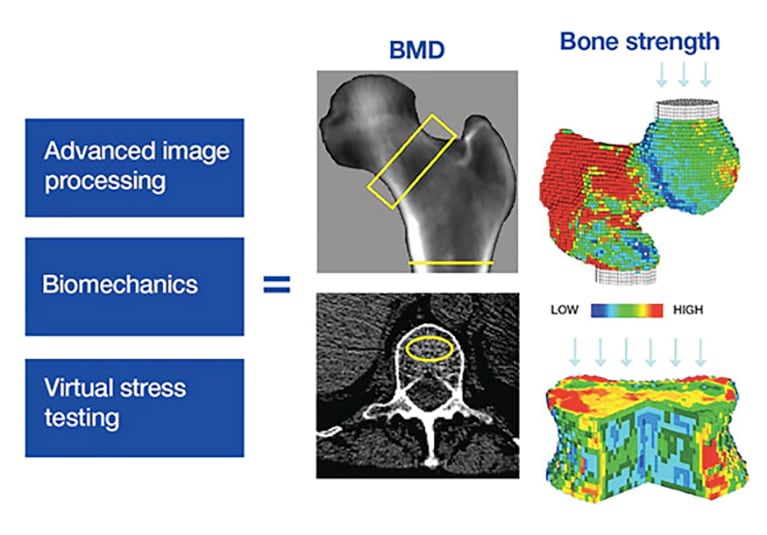
Quality control methods in musculoskeletal tissue engineering: from imaging to biosensors

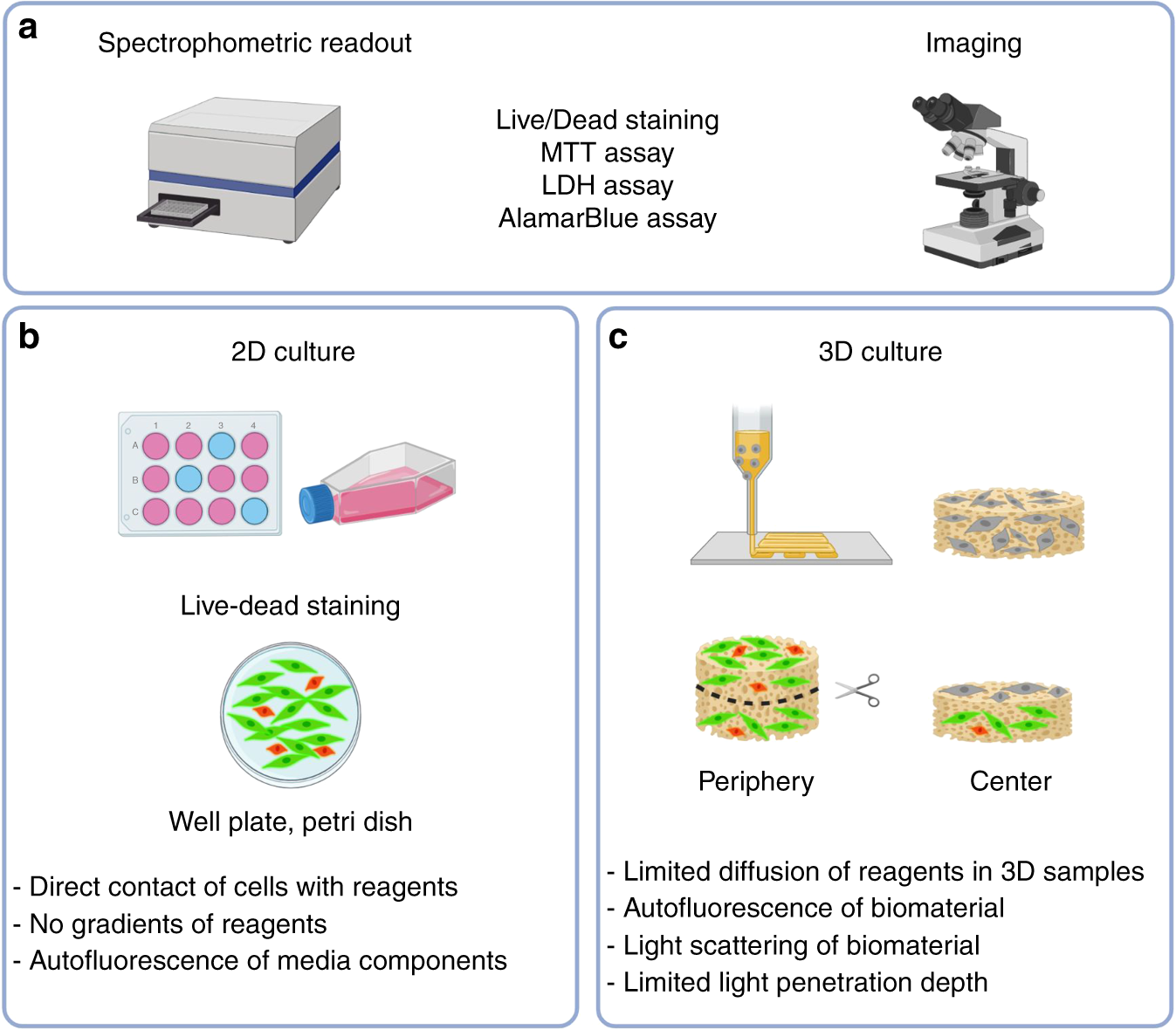
In vitro and in vivo toxicity assays. (A) In vitro cytotoxicity assay

Genotoxic and Cytotoxic Safety Evaluation of Papain (Carica papaya L.) Using In Vitro Assays
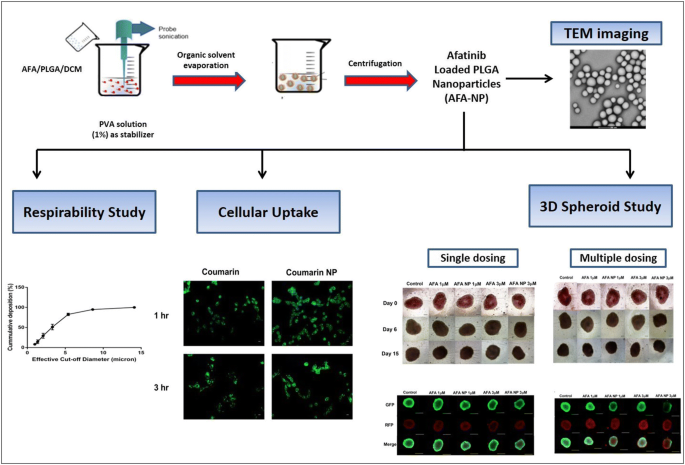
Afatinib-loaded inhalable PLGA nanoparticles for localized therapy of non-small cell lung cancer (NSCLC)—development and in-vitro efficacy
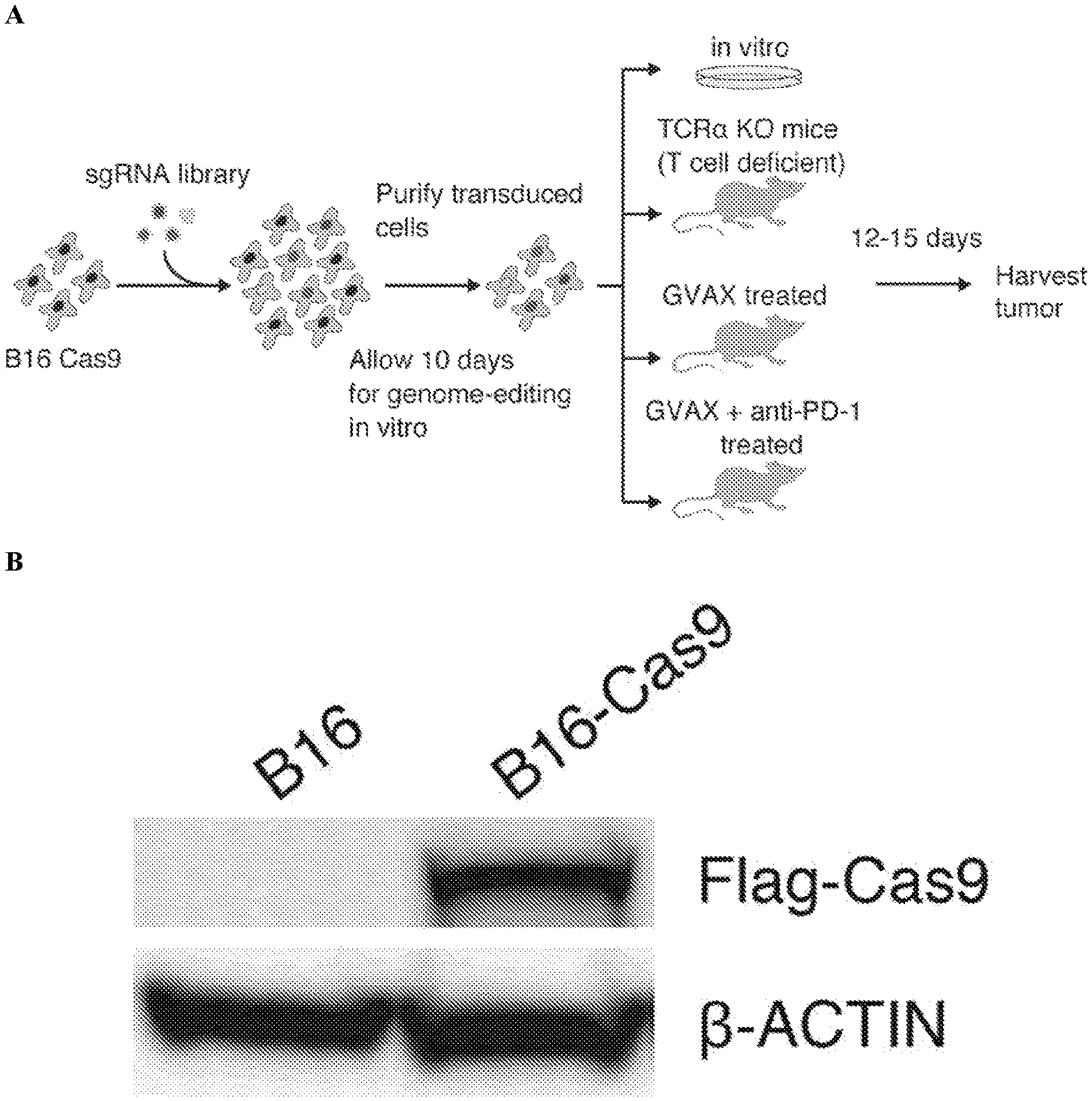
MODULATING dsRNA EDITING, SENSING, AND METABOLISM TO INCREASE TUMOR IMMUNITY AND IMPROVE THE EFFICACY OF CANCER IMMUNOTHERAPY AND/OR MODULATORS OF INTRATUMORAL INTERFERON Meyerson; Matthew ; et al. [Dana-Farber Cancer Institute, Inc.]

Fibrin Sealants from Fresh or Fresh/Frozen Plasma as Scaffolds for In Vitro Articular Cartilage Regeneration
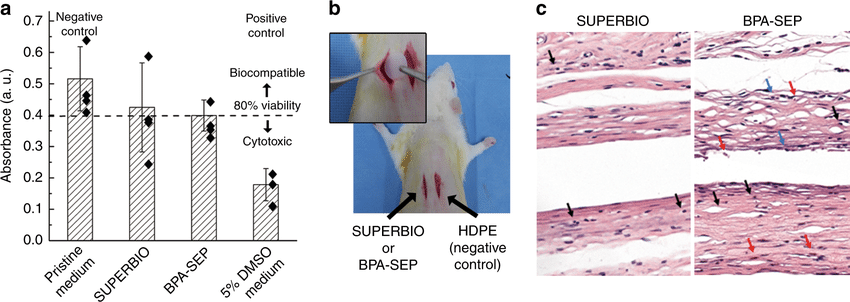
In vitro and in vivo biocompatibility tests. a In vitro cytotoxicity
de
por adulto (o preço varia de acordo com o tamanho do grupo)