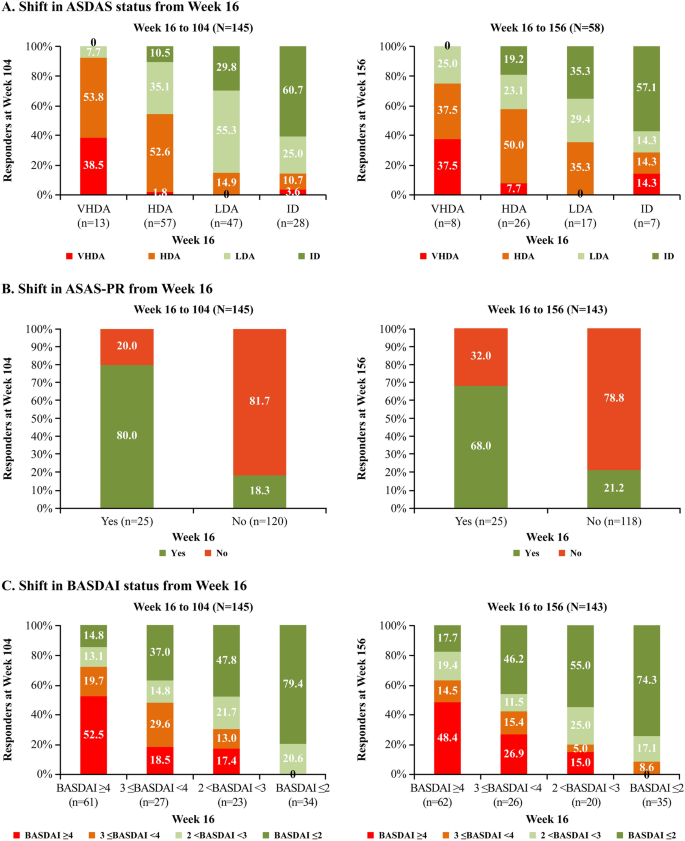
ASAS-HI improvement ≥30%, ASDAS LDA status and ASAS40 response
Descrição

Treating to Target(s) With Interleukin-17 Inhibitors - Charles W. Lynde, Jennifer Beecker, Jan Dutz, Cathy Flanagan, Lyn C. Guenther, Wayne Gulliver, Kim Papp, Proton Rahman, Dalton Sholter, Gordon E. Searles, 2019

Changes from baseline in ASDAS and BASFI over time. Dashed line: all
Disease Control Data, Ankylosing Spondylitis

Baseline characteristics of patients included in the two treatment arms
Achievement of Remission Endpoints with Secukinumab Over 3 Years in Active Ankylosing Spondylitis: Pooled Analysis of Two Phase 3 Studies

PDF] ASAS40 and ASDAS clinical responses in the ABILITY-1 clinical trial translate to meaningful improvements in physical function, health-related quality of life and work productivity in patients with non-radiographic axial spondyloarthritis

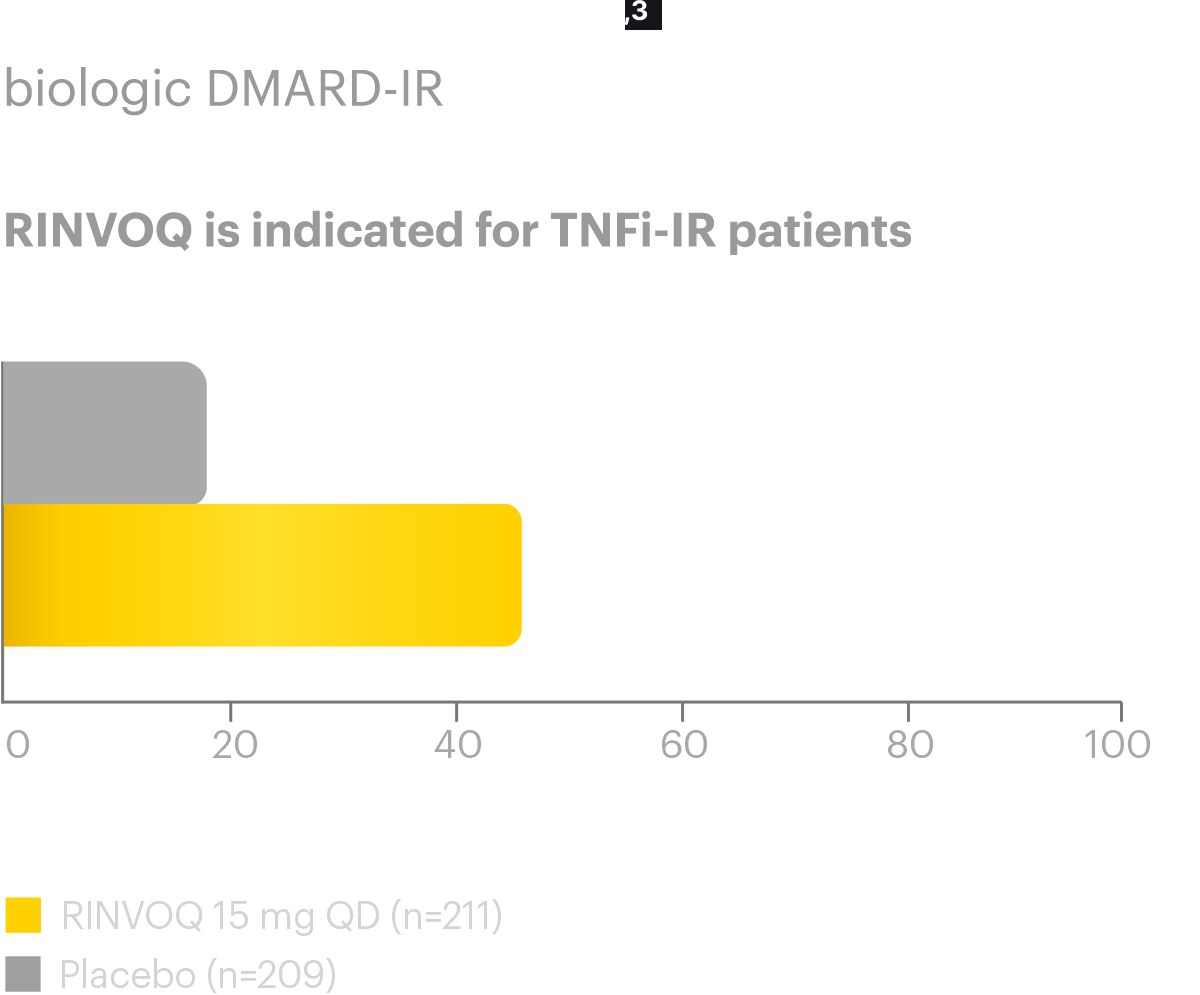
Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: a double-blind, randomised, placebo-controlled phase 3 trial. - Abstract - Europe PMC
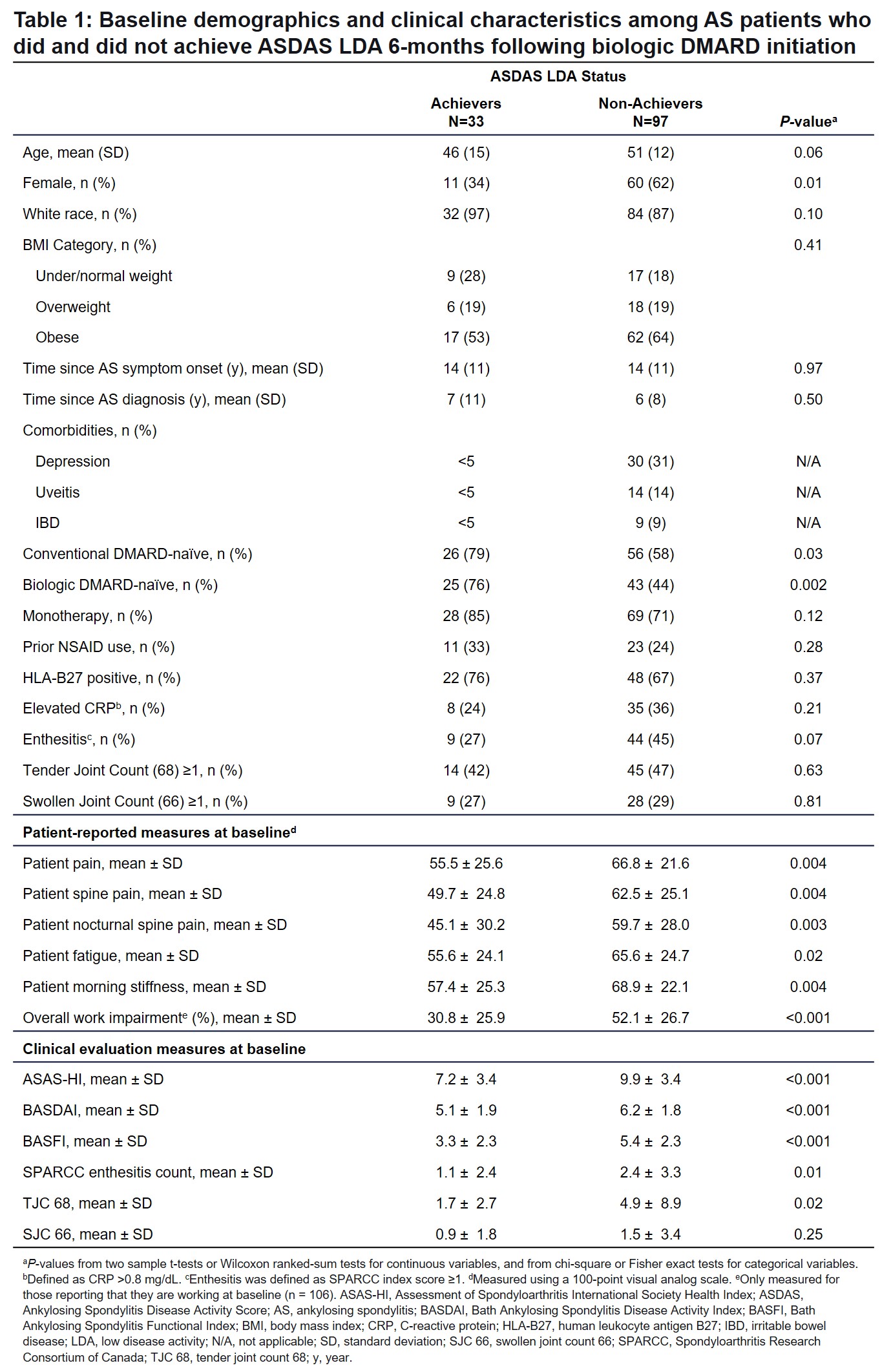
Impact of Achieving ASDAS LDA on Disease Activity and Patient-Reported Outcome Measures Among Patients with Ankylosing Spondylitis Treated with Biologic DMARDs - ACR Meeting Abstracts
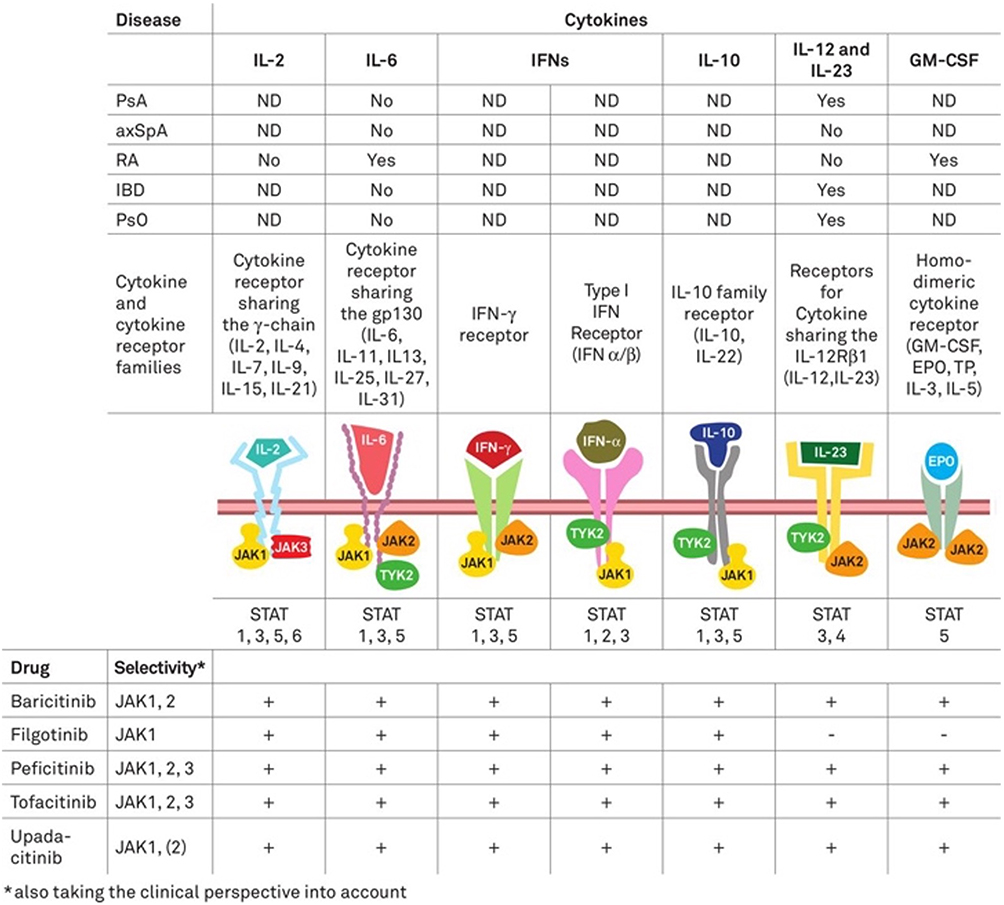
Management of axial spondyloarthritis
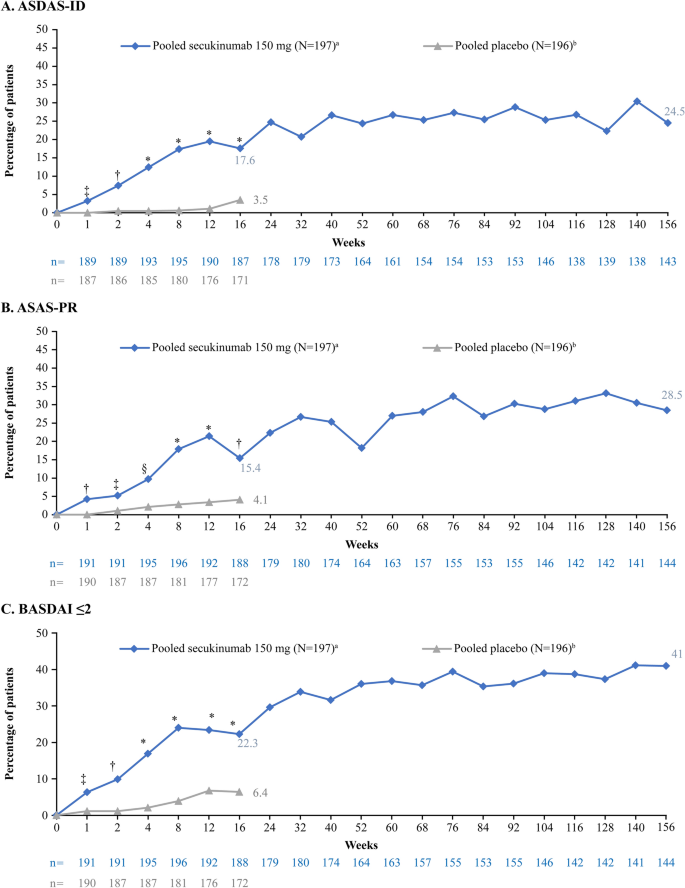
Achievement of Remission Endpoints with Secukinumab Over 3 Years in Active Ankylosing Spondylitis: Pooled Analysis of Two Phase 3 Studies
SIMPONI ARIA® Ankylosing Spondylitis: ASAS Response Rates
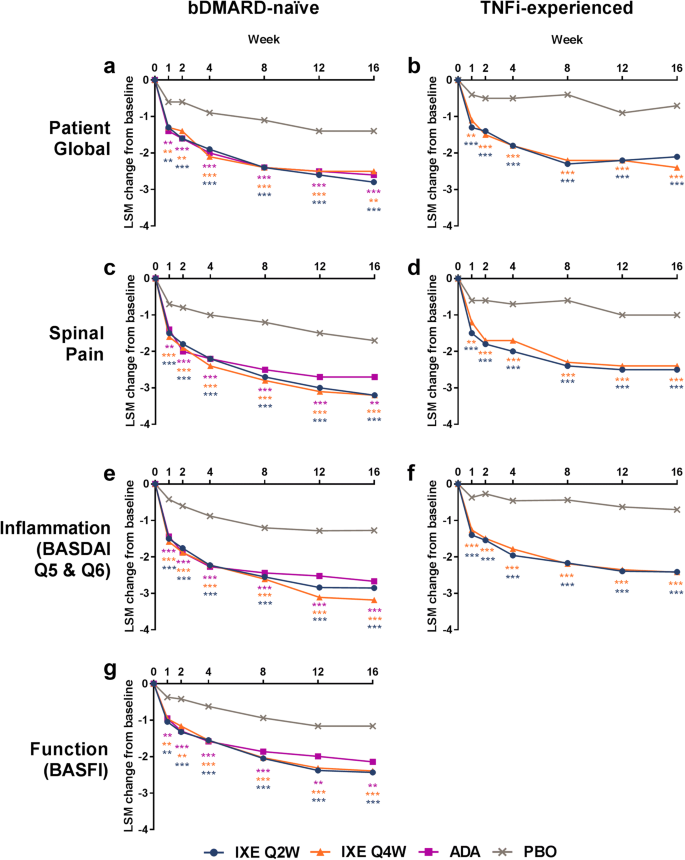
Translating Improvements with Ixekizumab in Clinical Trial Outcomes into Clinical Practice: ASAS40, Pain, Fatigue, and Sleep in Ankylosing Spondylitis

ARA Abstracts - 2020 - Internal Medicine Journal - Wiley Online Library
de
por adulto (o preço varia de acordo com o tamanho do grupo)