18F-fluciclovine-PET/CT imaging versus conventional imaging alone to guide postprostatectomy salvage radiotherapy for prostate cancer (EMPIRE-1): a single centre, open-label, phase 2/3 randomised controlled trial - The Lancet
Descrição

Randomized Trial of Conventional Versus Conventional Plus Fluciclovine (18F) Positron Emission Tomography/Computed Tomography–Guided Postprostatectomy Radiation Therapy for Prostate Cancer: Volumetric and Patient-Reported Analyses of Toxic

Fluorine-18-Labeled Fluciclovine PET/CT in Primary and Biochemical Recurrent Prostate Cancer Management

PDF) Impact of 18F-fluciclovine PET/CT on salvage radiotherapy plans for men with recurrence of prostate cancer postradical prostatectomy

Changes in Management After 18F-DCFPyL PSMA PET in Patients Undergoing Postprostatectomy Radiotherapy, with Early Biochemical Response Outcomes
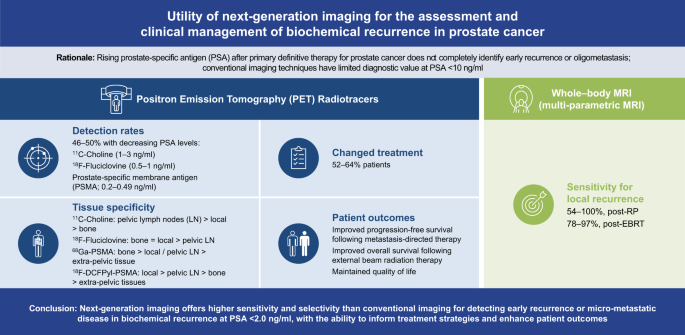
Application of next-generation imaging in biochemically recurrent prostate cancer
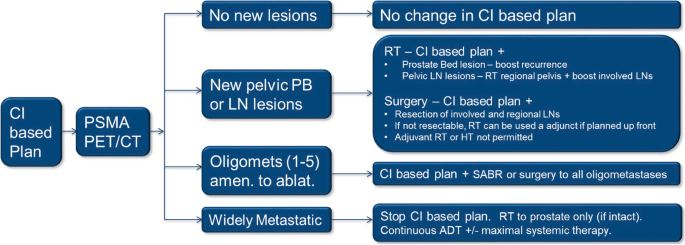
PSMA PET/CT guided intensification of therapy in patients at risk of advanced prostate cancer (PATRON): a pragmatic phase III randomized controlled trial, BMC Cancer

Fluorine-18-Labeled Fluciclovine PET/CT in Primary and Biochemical Recurrent Prostate Cancer Management
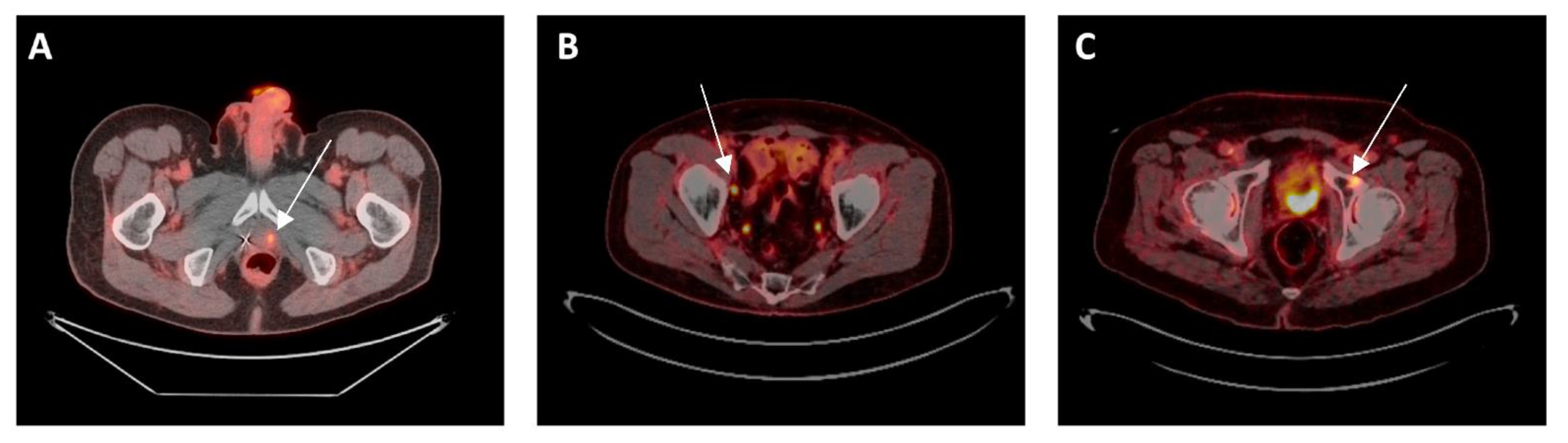
Cancers, Free Full-Text
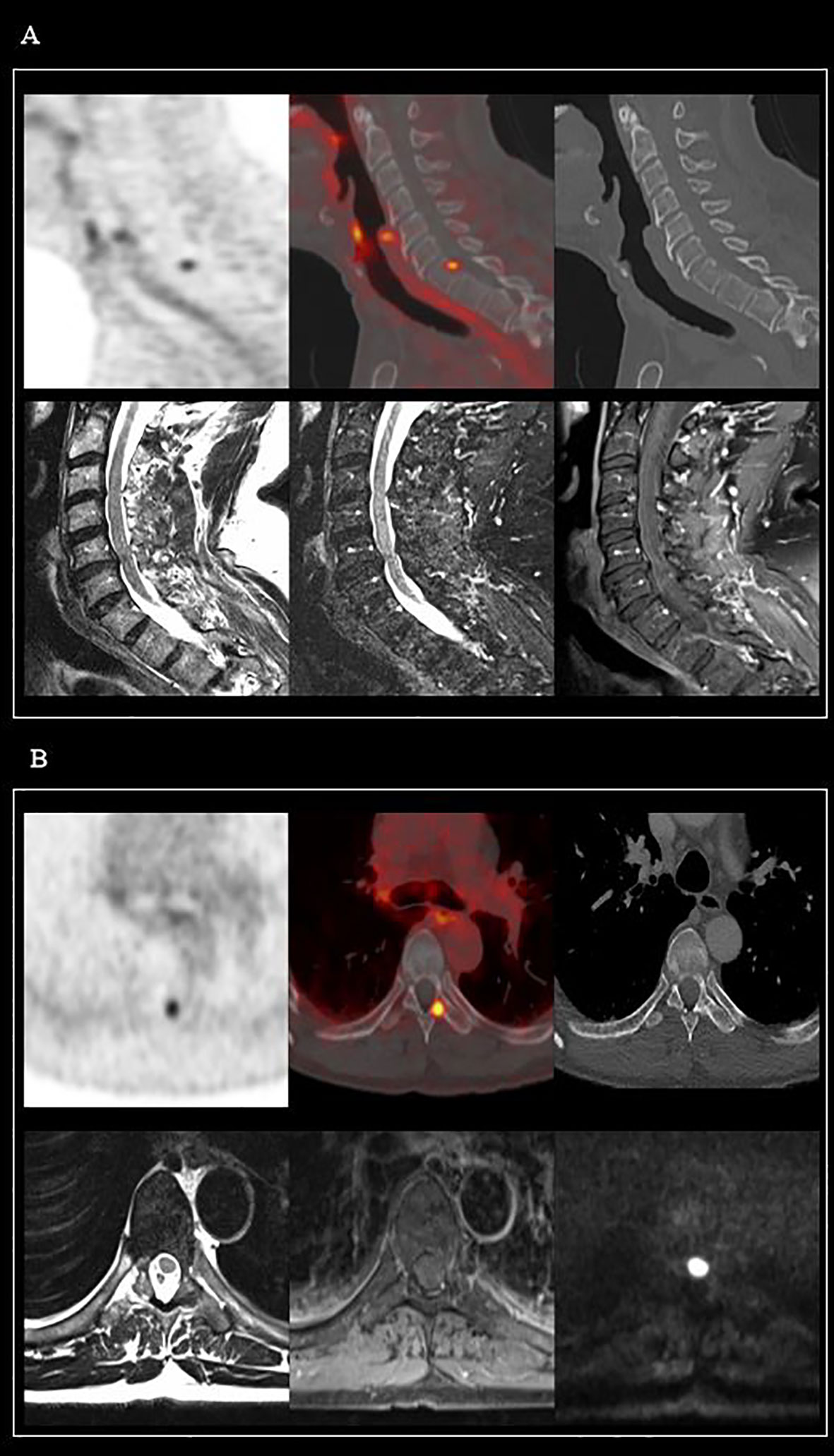
Frontiers PET/CT-Based Salvage Radiotherapy for Recurrent Prostate Cancer After Radical Prostatectomy: Impact on Treatment Management and Future Directions
de
por adulto (o preço varia de acordo com o tamanho do grupo)







